One of my long standing interest has been the organization the circuitry of the neostriatum. Over the last couple of decades in vitro physiological and anatomical studies by us and other showed that striatal interneurons form a circuitry of comparable complexity to homologous systems of cortical structures.
Three features of this system emerged from these investigations that provides clues to the functioning of striatal interneurons:
First, the circuit comprises a large number of physiologically distinct cell types. Currently, this includes up 9 different GABAergic and 2 types of cholinergic interneurons (CINs). Figure 1, shows a simplified circuit diagram
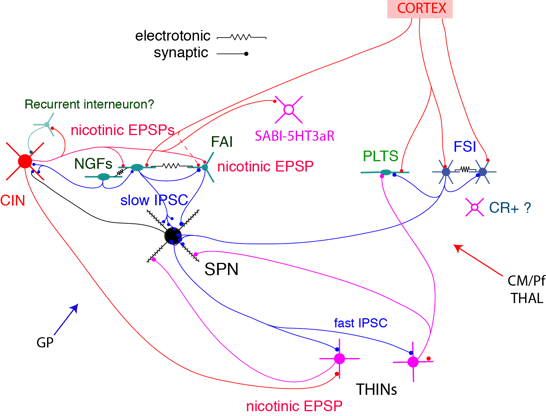
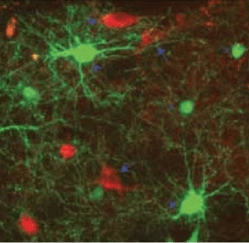
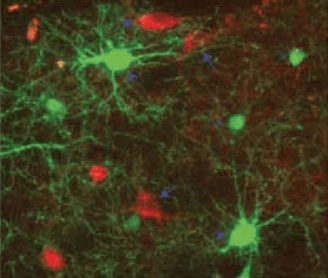
Neostriatal interneuron circuit
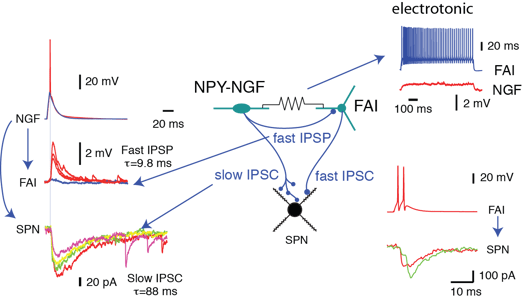
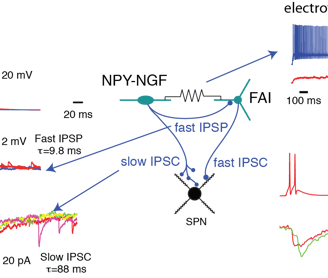
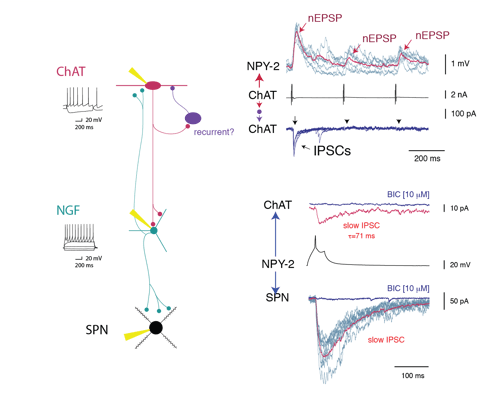
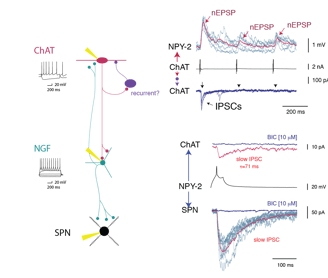
Second, the connectivity is highly cell type specific. For example, FSIs appear to be a sub-system of their own, since they have little or no connections with other interneurons. An even more striking example is the inhibition exerted by single axons of NGF cells (Fig.2), which involve GABAA-slow IPSPs with SPNs but conventional fast IPSCs with fast adapting interneurons (FAI).
Figure 1
Third, GABAergic and cholinergic interneurons form an integrated system in which many interneurons receive strong synaptic excitation through nicotinic receptors (Fig. 1,3).
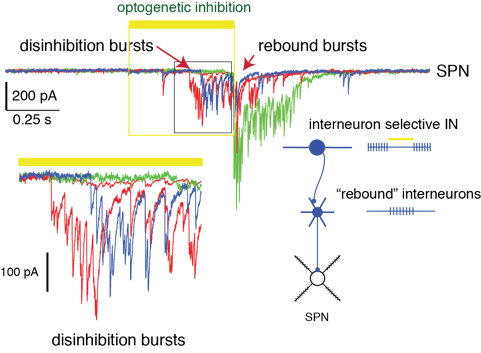
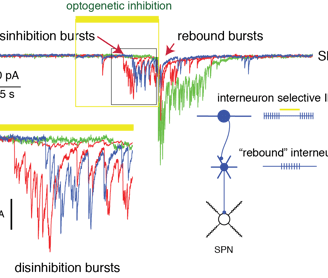
Interneuron targeting interneurons and complex internal dynamics of the network. In transgenic CR-Cre mice the targeted cells are clonally related subtypes of interneurons. Optogenetic inhibition of these cells induces a burst of large amplitude IPSCs in SPNs, indicating that a population of Cre- interneurons is relieved from tonic inhibition resulting in intrinsically driven activity (Fig.3)
CINs, targeted with ChR2-mCherry, NGF and PLTS interneurons expressing GFP in NPY-GFP/ChAT-Cre mice
Evolutionary archeology in the nervous system
Interneurons and their circuits share many notable similarities with the organization of simple neuronal systems already present in primitive Arthropods and even in Cnidaria. These characteristics include a large diversity of cell types represented in small numbers, utilization of neuropeptide and predominantly inhibitory neurotransmission, absence of dendritic spines and associated plasticity, stereotypical intrinsic oscillatory network activity, extensive electrotonic coupling and others. It is tempting to hypothesize that today's interneuron networks, which exhibit a striking degree of homology across different brain areas (cf. hippocampal and striatal systems) are evolutionarily preserved from early networks responsible for simple forms of innate behavior, such as digestive patterns and motor behavior Interestingly, striatal interneurons are associated with neuropsychiatric conditions with symptoms that show apparently intrinsic periodicities over different time scales. Taken together with the ancient origin of the basal ganglia we hypothesize that intrinsically periodic aspects of behavior in higher animals, such as activity-rest transitions may be intrinsically biased by intrinsic activity of interneurons.
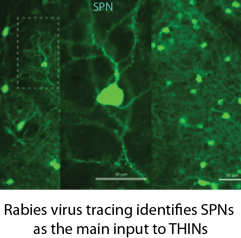
Another important feature of the connectivity was revealed by rabies virus tracing of synaptic inputs to THINs. These experiments showed that these interneurons receive dense innervation from projection cells which is specific to this class of GABA neurons.